THE UNITED REPUBLIC OF TANZANIA

PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
NJOMBE REGION
FORM SIX PRE-MOCK EXAMINATION
CODE: 132/2 CHEMISTRY 2
(For both school and private candidates)
TIME: 3:00 HRS Friday, 22nd August 2023 A.M
![]()
INSTRUCTIONS
1. This paper consists of six (06) questions.
2. Answer only five (05) questions.
3. Each question carries twenty (20) marks.
4. Mathematical tables and non-programmable calculators may be used.
5. Cellular phones and any unauthorized materials are not allowed in the examination room.
6. Write your examination number on every page of your answer booklet(s)
7. For calculations you may use the following constants
• Universal gas constant, R=8.314JK-mol- or 0.0821K-atm mol-K-dm3
• Velocity of light, C=3.0 ×108m/s
• Standard temperature=273K
• GMV=22.3dm3
• 1Faraday = 96500C
• Standard pressure=760mmHg = 1.0 ×105N/M2 = 1 atm
| QUESTION NUMBER | QUESTION ATTEMPTED PUT A TICK(V) | FOR EXAMINER’S USE ONLY | |
| MARKS | EXAMINER’S SIGNATURE | ||
| 1 |
|
|
|
| 2 |
|
|
|
| 3 |
|
|
|
| 4 |
|
|
|
| 5 |
|
|
|
| 6 |
|
|
|
| TOTAL MARKS |
|
|
|
| CHECKER’S INITIALS |
| ||
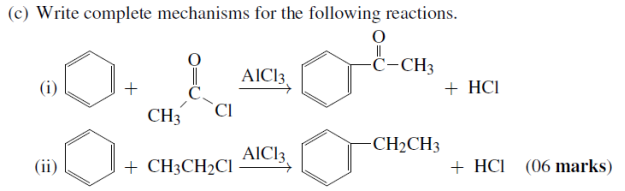
01. (a) (i) What does the term disprotonation means?
(ii) The reaction between dichromate (vi) Ions and chloride Ions in Acidic solution yield chlorine and chromium (iii) Ions as shown in the following unbalanced equation:
Cr2O7(aq) + Cl-(aq) → Cr3+(aq) + Cl2(g)
Derive the balanced Half – reaction equations and over all Net Equation for this redox reaction.
(b)A Form five student was trying to balance the equation then he noticed that single equation tends to loose and gain an electron simultaneously. State which of the following equation potray such behavior.
(i) CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
(ii) Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
(iii) Cr2O72-(aq) + 2OH-(aq) → 2CrO4 + H2O(l)
(iv) O3(g) + NO(g) → O2(g) + NO2(g)
(c) Identify the oxidizing agent, reducing agent, the substance being oxidized and the substance being reduced in 01 (b) (i-iv)
(d) Calculate the Emf of the following
Pb/Pb2+aq(1×10+3m)// Cu2+ (1×10-2m)/ Cu(s)
Given the following standard reduction potentials Pb2+/Pb Eθ= - 0.126v: al2+/ Cu, Eθ = 0.34v.
02. (a) (i) State the distribution law.
(ii) Distribution of solute particles in some given solvents can be governed by different condition and principles. Briefly explain the conditions that govern such distribution.
(b) (i) Calculate the percentage by mass of bromo benzene (C6H5Br) in the distillate when a mixture of bromo benzene and water distills at 65˚C in the steam. The vapour pressure of bromo benzene and water are 1.59 × 104 and 8.50 × 104Nm-2 respectively.
(ii) Heptane (C7H16) and Octane (C8H18) form an ideal solution at 373K, the vapour pressure of pure heptane and octave were 105.2Kpa and 46.8 Kpa respectively. Calculate the vapour pressure of the mixture of 26.0g of heptane and 35.0g of octane.
(c) Compound P has a partition coefficient of 4.00 between the Ethoxyethane and water. Given that 2.0g of P is obtained in solution, in 50cm3 of water, calculate the mass of P that can be Extracted from the Aqueous solution by
(i) 50cm3 of Ethoxyethane
(ii) Two successive extractions of 25cm3 of ethoxyethane each
(d) To a solution containing 0.1m Cl- and 0.01m CrO42- a solution of AgNO3 is added slowly.
(i) Which salt will precipitate first between AgCl and AgCrO4? Show clearly how you arrived to your answer.
(ii) Find the concentration of the Ion that will precipitate first at the time the second Ion will start precipitating.
Use Ksp (AgCl) = 2.72 × 10-10 and Ksp (Ag2CrO4) = 2.4 × 10-12
03. (a) Rate of chemical reaction can proceed fast or slow depending on some factors in a given chemical reaction. Briefly explain. (b) consider the following reaction
H2(g) + I2 (g) → 2HI(g)
If the rate constant K at 310˚C and 420˚C is 1.3 × 10-3dm-3mol-s- and 6.3 × 10-3dm 3mol-s- respectively, what is the activation energy Ea for the reaction?
(c) The reaction of nitric oxide with hydrogen at 1280˚c is given by the equation
2NO2 (g) + 2H2 (g) → N2 + 4H2O
This reaction was studied and the following results were obtained
| (NO2) M | (H2) M | RATE (M/S) |
| 0.42 | 0.7 | 0.41 |
| 0.84 | 0.7 | 0.82 |
| 0.84 | 1.4 | 0.82 |
(i) Determine the order with respect to each reactant and the overall order of the reaction
(ii) Establish the rate law for the reaction
(iii) Calculate the rate constant
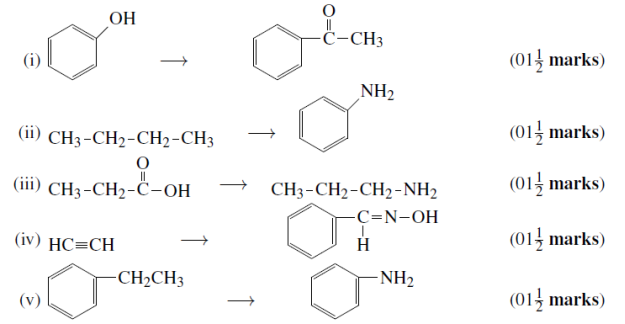
03. a) An aromatic compound D (C8H8O) give a positive result with 2.4 dinitro – phenyl hydrozone but gives yellow precipitate of compound E treatment with iodine and sodium solution. Compound D give negative Tollens or Fehling test. On drastic oxidation with KMnO4 forms a carboxylic acid F (C7H6O2) which is also formed along with the yellow compound in the above reaction. Identify the structure D, E, and F also write all chemical reaction involved.
(b) A compound A of Molecular formula of C3H7O2N On reaction with iron and concentrated hydrochloric acid give the compound B of molecular formula C3H8O. Compound C Gives Effervescence with sodium on oxidation with CrO3 compound C given saturated aldehyde containing three carbonic atoms deduce the structure of A, B and C
04. (a) (i) Generally extraction of metals from their ores involves four stages. Obtaining the ore, concentating the ore, concentrating the compound of interest in the ore, chemical reduction and refining of the crude metal. Describe how Aluminium is extracted from its ore basing on these stages.
(ii) Show that the properties of aluinium suit its wide range use.
(b) Transitional elements show a variety of behaviours. With vivid example explain five (05) properties shown by such metals.
(c) Give the IUPAC name of the following
i. [Cr(H2O)4(NH3)2]Cl3
ii. [CoCl(NO2) (en)2]+
iii. K4[Fe(CN)6]
iv. [FeBr2(H2O)4]+
v. [CoCl2(NH3)4]3 [Cr(CN)6]
05. (a) (i) Arrange the following compound in the order of increasing acidic strength Phenol, phenylmethanol, 2-nitrobenzoic acid, benzoic acid
(ii) A compound with the following structural formula was extracted from certain yellow flowers H2N-CO-H2CHOH-CO-OH.
From your knowledge in organic chemistry give the product that will be formed when this compound is treated with
i)Nitrous acid (ii) Ethanol (iii) LiAlH4 (iv) Potassium dichromate (v) PCl5
(b) Show the initiation step and propagation step for the following Polymerization types for Polytetrafluoroethene (PTFE) polymer.
i. Free radical polymerization
ii. Cation polymerization
iii. An ionic polymerization
(c) Write the monomers forming the following polymers and show the polymerization method used
i. PVC
ii. Bakelite
iii. Teflon
iv. Nylon bile
v. Dacron
FORM SIX CHEMISTRY EXAM SERIES 71
FORM SIX CHEMISTRY EXAM SERIES 71
THE UNITED REPUBLIC OF TANZANIA

PRESIDENT’S OFFICE, REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
NJOMBE REGION
FORM SIX PRE-MOCK EXAMINATION
CODE: 132/1 CHEMISTRY 1
TIME: 3:00 HRS Friday, 18th August 2023 A.M
![]()
INSTRUCTIONS
1. This paper consists of section A and B with a total of ten (10) questions
2. Answer ALL questions in section A and two questions from section B.
3. Mathematical tables and non-programmable calculators may be used.
4. Cellular phones and any unauthorized materials are not allowed in the examination room.
5. Write your examination number on every page of your answer booklet(s) 6. The following information may be used.
a) Universal gas constant, R=8.314JK-1mol-1 or 0.0821 atmdm3mol-1
b) Rydberg constant RH=1.09678 x 107m-1
c) Standard pressure =760mmHg =1.0X10-5Nm2
d) A mass of electron =9.11x10-31kg
e) Density of water =1g/cm3
f) Velocity of light, C= 3.0 X 108 m/s
g) Avogadro’s number = 6.02x1023
h) Planks constant, h=6.63 x 10-34Js
i) Atomic masses
H=1, C=12, O=16, N=14, Cl= 35.5, Br=80, Na=23
| QUESTION NUMBER | QUESTION ATTEMPTED PUT A TICK(V) | FOR EXAMINER’S USE ONLY | |
| MARKS | EXAMINER’S SIGNATURE | ||
| 1 |
|
|
|
| 2 |
|
|
|
| 3 |
|
|
|
| 4 |
|
|
|
| 5 |
|
|
|
| 6 |
|
|
|
| 7 |
|
|
|
| 8 |
|
|
|
| 9 |
|
|
|
| 10 |
|
|
|
| TOTAL MARKS |
|
|
|
| CHECKER’S INITIALS |
| ||
SECTION A (70 MARKS)
Answer all questions from this section.
1. (a)Why does hydrogen spectrum have large number of lines despite the fact that hydrogen has one electron? (02 marks)
(b) Calculate the wavelength of an alpha particle having a mass of 6.7 x 10-25 kg moving with a speed of 1000m/s. (04 marks)
(c) Naijongoro a chemistry teacher at Muungano secondary school told his student that “Dalton atomic theory is primitive” supports Mr. Naijongoro using four points. (04 marks)
2. (a) Write down the molecules or ions that undergo the following hybridization scheme;
i. sp3d
ii. sp3d2
iii. sp3 (03 marks)
(b) CO2 is a non- polar molecule while H2O is polar molecule. Explain. (03 marks)
(c) Makene a form five student was arguing that double bond is a weaker than single bond because double bond has a weak Pi bond. But Anna happened to educate Makene that in double bond there are two bonds, a strong sigma bond and weak pi bond while in single bond there is only one bond, which is strong sigma bond. Using four points justify that sigma bond is different from Pi bond (04 marks)
3. (a) Define the following
i. Ebullioscopic constant
ii. Van’t Hoff’s factor
iii. Colligative properties (03 marks)
(b)The vapour pressure of water at 500C is 92.5mmHg. At the same temperature a solution containing 9.14g of urea in 150g of water has a vapour pressure of 90.8mmHg. Determine the molecular mass of urea. (04 marks)
(c) What is the boiling point of a solution made by dissolving 45.0g of NaCl in 500g of water (ebulliscopic constant, Kb=0.512) (04 marks)
4. (a) Explain the following.
i. Heat of neutralization
ii. Heat of solution (02 marks)
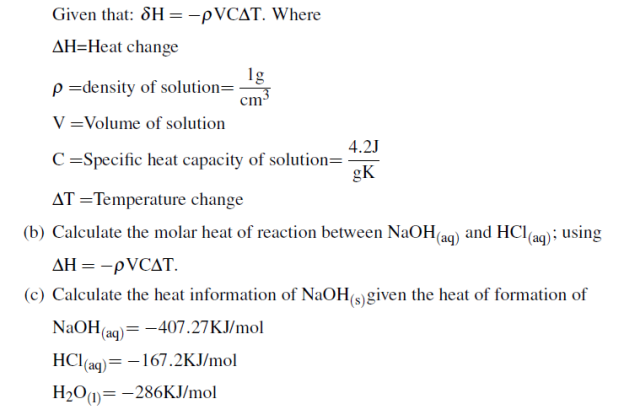
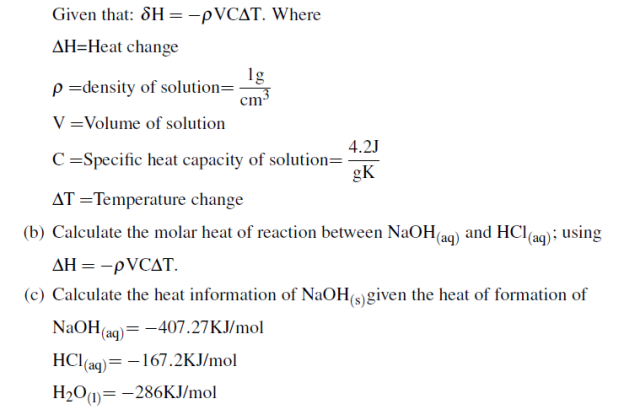
(b) 1. 5g of ammonium nitrate was added to 35.0g of water in a plastic beaker and stirred until the salt dissolved. The temperature of the solution dropped from 22.70C to 19.40C basing on the given information respond to the following questions.
i. Is the process endothermic or exothermic? Explain
ii. Calculate the heat of solution ammonium nitrate in KJ/mol given that specific heat capacity of water=4.184J/g0C and density of water is =1g/ml (04 marks)
(C) Calculate the heat of formation of ethyl alcohol (C2H5OH) if the heat of combustion of ethyl alcohol is -1380.7KJ/mol and heat of formation of CO2 and H2O are 393.9KJ/mol and -286KJ/mol respectively. (04 Marks)
5. (a) Aluminium oxide is said to be amphoteric. Explain this fact by aid of chemical Equation. (03 marks)
(b)Iron III carbonate never exists. Explain this statement. (01 marks)
(c)Explain the following with the aid of chemical reaction if applicable.
i. MgCl2.6H2O when heated can never give out unhydrous MgCl2
ii. CuCl2 solution is acidic to litmus paper
iii. Fe3O4 is called mixed oxide. (06 marks)
6. (a) Explain these concept
i. Dynamic equilibrium
ii. Irreversible reactions (03 marks)
(b) At 773K, the reaction between gaseous nitrogen and hydrogen to form ammonia gas has a KC =6.0 x10-2dm6mol-2. Compute the value of KP for this reaction. (03 marks)
(c) A mixture of CO and steam consisting of 0.25mol each constituent is placed in 500ml flask and the mixture heated up to 900K. The reaction equations is
CO (g)+H2O(g) → H2(g)+ CO2( g).
What is the composition of the equilibrium mixture if the KC for this reaction is 1.56? (04 marks)
7. (a)Suppose you are invited by a village chairman Mzee Yakwamba at Igongolo village to educate people about the importance of ozone layer in the atmosphere. Briefly explain with the aid of equations where necessary on the formation of ozone layer, primary causes of ozone layer destruction and three effects of ozone layer depletion. (08 marks)
(b)How can an elemental sulphur be efficiently converted to sulphuric acid in the soil? (02 marks)
SECTION B (30 MARKS)
Answer two (02) questions in this section.
8. (a) (i) By using the chemical equation explain why tertiary haloalkane can not undergo SN2 reaction mechanism
(ii) Benzene is more reactive than nitrobenzene while methylbenzene is more reactive than benzene. Explain this observation. (04 marks)
(b) Explain which substituent entered in the benzene ring first
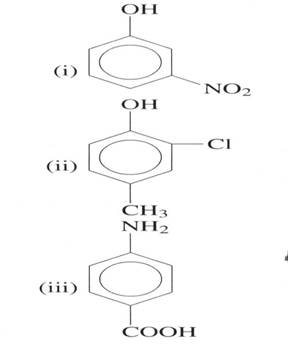 (03 marks)
(03 marks)
(c) Convert CH3CH3 to 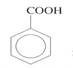 in more than three steps. (03 marks)
in more than three steps. (03 marks)
(d) From the scheme below identify the molecules V, W, X, Y, and Z
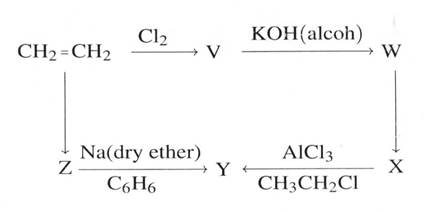 (05 marks)
(05 marks)
9. (a) Explain briefly the physical significance of the van der waals’ parameters, “a” and” b” (02 marks)
(b) Explain the following.
i. Aerated water bottles are kept under water during summer
ii. The size of weather balloon becomes larger and larger as it ascends into high attitudes. (03 marks)
(c) Explain briefly the application of Avogadro’s law in life situations. (03 marks)
(d) A hydrocarbon contains 10.5g of carbon per g of hydrogen. 1 litre vapour of hydrocarbon at 127oC and 1atm pressure weighs 2.8g. Find the molecular formula of a hydrocarbon. (07 marks)
10. Predict the major product(s) for each of the following reactions
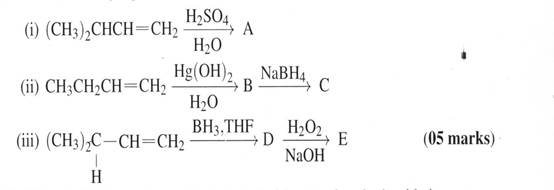 (b) When 1,2-dibromodecane was treated with potassium hydroxide in aqueous ethanol, it yields a mixture of three isomeric compounds of molecular formula C10H19Br. Each of these compounds was converted to dec-1-yne on reaction with sodium amide in dim ethyl sulphoxide. Identify the three compounds. (06 marks)
(b) When 1,2-dibromodecane was treated with potassium hydroxide in aqueous ethanol, it yields a mixture of three isomeric compounds of molecular formula C10H19Br. Each of these compounds was converted to dec-1-yne on reaction with sodium amide in dim ethyl sulphoxide. Identify the three compounds. (06 marks)
(c) Explain briefly the preparation of acetylene (ethyne) by; -
i. Pyrolysis of natural gas. ii. Action of water on calcium carbide. (04 marks)
FORM SIX CHEMISTRY EXAM SERIES 70
FORM SIX CHEMISTRY EXAM SERIES 70
MINISTRY OF EDUCATION, SCIENCE AND TECHNOLOGY
TABORA BOY’S SECONDARY SCHOOL
FORM SIX TERMINAL XAMINATION
132/1 CHEMISTRY 1
Time: 3 Hours , 2023
Instruction
- This paper consists of fourteen(14) questions in Section A, B and C
- Answer four (4) questions from section A and three (3) questions from each of sections B and C
- Each question carries ten(10) marks
- Mathematical tables and non- programmable calculators may be used.
- Cellular Phones are not allowed in the Examination room.
- For your calculations you may use the following constants
- Rydberg constant
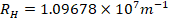
- Gas constant,
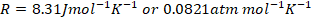
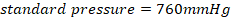
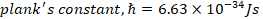
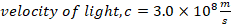
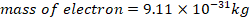
- (a) State the rules which govern the pairing of electrons in orbitals of an atom which is in ground state
(b) Give the name of a geometrical structure and one example of the molecule formed from the following hybridized atomic orbtals
(i) sp3 hybridized orbitals
(ii) sp2 hybridized orbitals
(iii) d2 sp3 hybridized orbitals
(c) Calculate the energy associated with an electron with an electron moving in an orbital of principle quantum number n=2
- (a) What do you understand by:-
- Real gas
- Critical pressure?
(b) State:
(i) Graham’s law of diffusion
(ii) The equilibrium law.
(c) 200cm3 of oxygen gas takes 250 seconds to diffuse through a porous diaphragm. Under identical conditions, 200cm3 of an unknowns gas T takes 177 second to diffuse. Calculate the relative molecular mass of the unknown gas
- (a) Define:
- Molarity
- Molality
(b) Calculate the molarity and molality of a concentrated nitric acid having a density of 1.4g/cm3 containing 75% nitric acid by mass.
4. (a) State:
(i) A homogenous catalyst.
(ii) A heterogeneous equilibrium
(iii) Autocatalyst.
(b) Deduce the relationship between Kc and Kp for the following gaseous equilibria:
![]()
(c) Consider the following equilibrium:
![]()
5. (a) State Avogadro’s
(b) A poisonous constituent of tobacco was isolate and purified. Subsequent
combustion analysis of a 3.0g of the sample yielded 8.04g of CO2, 2.66g of water and 0.569g of nitrogen. If the relative molecular mass of the compound was 150, determine the:
(i) Molecular formula of the compound
(ii) Number of molecules of the compound present in 3.05g of the sample.
6. (a) Cobalt, iron and manganese are transition meatals
(i) What is meant by the term transition metal?
(ii) Explain in terms of electronic configuration why Fe2+ can readily be
oxidized to Fe3+ while Mn2+ cannot be oxidized to Mn3+
(b) Cobalt can form complex compounds of formulae:
A = [Co(NH3)4Cl2]+
B = [Co(NH3)3Cl3]-
C = [Co(NH3)4Cl2]Cl
- What is the oxidation state of cobalt in compound A,B and C?
- Give the IUPAC names of the three compound A,B and C
- What will be observed if to a solution of these three complexes A,B and C in different test tubes, silver nitrate solution is added?
(c) Chromium forms a complex chloropentaamine chromium (III) ion.
- Write the formula of this complex
- Give the coordination number of this complex
- Write the geometrical structure of this complex
7. (a) Define
- Periodicity
- Valency electrons
- Electronegativity
- Atomic radius
(b) (i) State the periodic law according to Mendelef
(ii) What were the usefulness of the Mendelef’s periodic table?
- What were the defects of the Mendelef’s periodic table?
(c) Electronic affinity value for periodic three (3) elements are as follows:
| Element | Na | Mg | Al | Si | P | A | Cl | Ar
|
| EA(J/mol) | -75 | +42 | -47 | -134 | -75 | -200 | -354 | +29
|
From the electron affinity energy value in the table answer the following questions:
- Why are the electron affinity energy value of Magnesium and Argon positive (endothermic) unlike those of the other periodic three (3) members?
- Why is the electron affinity value of phosphorus less exothermic than that of silicon despite close to group VII A elements which have most exothermic value of electron affinity?
8. (a) Write balanced chemical equations for the following reaction
- Excess carbon dioxide is bubbled in sodium sulphate solution
- Excess sulphuric acid is added to sodium sulphate solution
- A white precipitate is observed when sodium sulphate solution is added to barium chloride solution in the presence of hydrochloric acid
(b) (i) What is the difference between hydrolysis and hydration?
(ii) Give two (2) supporting reaction equations to show clearly the contrast
between hydrolysis and hydration.
(c) Elements is group I and II are normally extracted by electrolysis of their fused chlorides. Explain why?
9. (a) Define the terms hybridization
(b) By applying the knowledge of hybridization explain the following:
- A carbon atom has got only two unpaired electrons yet, it can form four covalent bonds with chlorine atoms to form a carbon tetrachloride molecule.
- Beryllium has a pair of electrons in its outer most shell but it can form a beryllium molecule by means of sharing electrons with two chlorine atoms to form a beryllium chloride molecule.
(c) Give explanations to the following observations:
- Anhydrous aluminium chloride does not conduct electricity but its aqueous solution does
- An aqueous solution of aluminium chloride turns blue litmus red.
- A white precipitate tends to be formed which dissolves when carbon dioxide is bubbled to excess through calcium hydroxide solution
10. (a) Explain briefly the following terms
- Indicator
- Titrant
- End – Point
(b) Commercially available concentrated hydrochloric acid is an aqueous solution containing 38% HCl acid by mass and has a specific gravity of 1.18g/cm3
- Calculate its molarity.
- How many cm3 of concentrated hydrochloric acid are required to make 25cm3 of 0.01M HCl solution
11. (a) Name the following compound according to the IUPAC system:
- CH2(OH)CH2CH2OH
- CH3CH(OH)CH2Cl
- CH3CH(OH)CH(OH)CH3
(b) Compound x containing a carbon – carbon double bond would react with the following reagents:
- Y to form 2 – bromo – 2 – methlybutane
- Z to form (CH3)2CHC(OH)CH3)2
- W to form 2 – chloro – 2 – Methylbutane.
Name compound X and reagents Y,Z and W
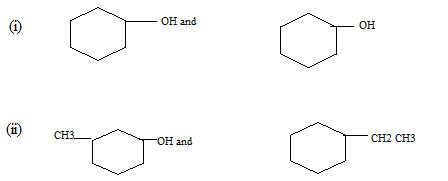
(c) Indicate a chemical test which may be used to distinguish the members of each of the following pairs. Indicate of the pair that gives the positive or greater reaction.
12. The following is a full structure formula of a certain organic molecule P. study
structure and then answer the questions that follow:
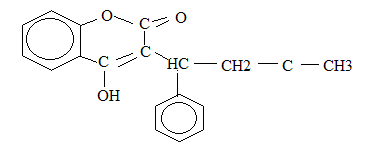
(a) If the molecule gives a positive iodoform test:
- What are the reagents and conditions for the test?
- Show on the formula which part of the molecule P gives the tri-iodomethane
(b) Indicate on the formula the part of the molecule which will react with 2, 4-
dinitrophenylhdrazine
(c) Explain whether P would give a positive reaction with [Ag(NH3)2]+
(d) If molecule P reacts with aqueous sodium hydroxide then:
- Name the group in P which is attached by sodium hydroxide
- Draw the structure of the product formed by the reaction.
13. With support of chemical reactions show how the following compounds can be prepared from ethanol as source of carbon atoms
- Benzene
- Ethly benzene
14. (a) What is ozonolysis?
(b) A hydrocarbon having a molar mass of 96gmol-1 and molecular formula C7H12 was ozonolysed and then hydrolysed in the presence of zinc. The products of ozonolysis were ethanol, propanone and glyoxal.
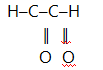
Determine the structure of the hydrocarbon and show ozonolysis of the
compound.
- Two isomeric hydrocarbon P and Q have molecular formula C9H12. On oxidation, P gives monocarboxylic acid, when treated with soda lime yields benzene. Q is oxidized to give tricarboxylic acid and can undergo nitration reaction to give two mono – nitro derivative.
- Write down the structural formula of P and Q
- Write a equation to show how Q is oxidized to give tricarboxylic acid
- Name the compound which is formed when P undergoes oxidation
Page 1 of 7
FORM SIX CHEMISTRY EXAM SERIES 51
FORM SIX CHEMISTRY EXAM SERIES 51
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
KILIMANJARO REGIONAL COMMISSIONER’S OFFICE
FORM SIX MOCK EXAMINATION
CHEMISTRY 2
INSTRUCTIONS
- This paper consists of six (06) questions
- Answer any five (05) questions.
- Each question carries twenty (20) marks.
- Mathematical table and non-programmable calculator may be used.
- Write your Examination Number on every page of your answer booklet(s)
- Cellular phones are not allowed in the examination room.
- For calculations you may use the following constants:
- Atomic masses: K=39, Ag=108, Br=80, Cl=35.5, Na=23, S=32, Ca=40, Ar = 40, Fe=56
- Atomic number: Al=13, O=8, Li=3, Cl=17, K=19, Cr=24, Cu=29, Fe=26,
Ca=20, Ag=47, Ne=10, Ar=18, Kr=36, Xe=54, Br=35, Mg=12, F=9, N=7
- Gas constant, R=0.0821 atmLmol-1K-1 or 8.314JMol-1K-1.
- (a)State partition law
(b) 25cm3 of 0.2 mol dm3 solution of copper (II) sulphate were mixed with 25cm3 of 1 mol dm-3 ammonia solution. When these solutions mix, a royal blue solution is made. This contains a complex ion, which we can write as Cu(NH3)x2+.The total 50cm3 of the solution were shaken with 50cm3 of trichloromethane. After giving time for equilibrium to be achieved, the two solutions were separated. The ammonia was extracted from the trichloromethane. After performing a titration it was found that 0.2 x 10-3 mol of ammonia was present in the trichloromethane. Taking the partition coefficient for ammonia between water and trichloromethane as 25.
- Calculate the number of moles of free ammonia in the orginal 50cm3 of the royal blue solution
- What is the total number of moles of ammonia in the trichloromethane and the 50cm3 of the royal blue solution?
- How many moles of ammonia were present before the complex ion was made?
- How many moles of ammonia had been used in joining with the copper (ii) ions?
- How many moles of copper (II) sulphate had been used
- Given that every 1mol of copper (II) sulphate contains 1 mol of copper (II) ions, how many moles of coper (II) ions had been taken?
- What is the ratio of moles of copper (II) ions to moles of ammonia molecules in the complex ion?
- What is the formula of the complex ion?
(c)(i)State Raoult’s law of vapour pressure
(ii)Benzene (C6H6) and toluene form a nearly ideal solution. At 313K the vapour pressure of pure benzene is 150mmHg and of pure toluene is 50mmHg. Calculate the vapour pressure of a mixture of these two liquids containing equal masses at the given temperature.
(d)A. 1.0g sample of Fe2O3 solid of 55.2 per cent purity is dissolved in acid and reduced by heating the solution with zinc dust. The resultant solution is cooled and made up to 100cm3. An aliquot of 25.0cm3 of this solution requires 17.0cm3 of 0.0167M solution of an oxidant for titration. Calculate the number of electrons taken up by the oxidant in the reaction of the above titration.
- (a)Distinguish between the following terms:
- Rate constant and rate law
- Elementary step and rate-determining step
- Molecularity and order of reaction
(b)The following results were obtained for the decomposition of nitrogen (v)
Oxide. 2N2O5(g) → 4NO2(g) + O2(g)
| Initial concentration of N2O5/moldm3 | Initial rate (mol/dm3/s) |
| 1.6 x 10-3 | 0.12 |
| 2.4 x 10-3 | 0.18 |
| 3.2 x 10-3 | 0.24 |
- What is the Order of reaction?
- Determine the rate constant for the reaction
- What is the initial rate of reaction when the concentration of N2O5 is 2.4 x 10-2 moldm-3?
(c)(i)The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350K to 400K. Calculate the activation energy for the reaction.
(ii)During the nuclear explosion, one of the products is 90Sr with half-life of 28.1 years. If lug of 90Sr was absorbed in the bones of a newly born baby instead of calcium, how much of it will remain after 10 years and 60 years if it is not lost metabolically?
(d)The decomposition of a certain insecticide in water follows first order kinetics with a rate constant of 1.45 year- at 285K. A certain amount of this insecticide is washed into a lake on June 1st leading to a concentration of 5.0 x 10-7gcm-3. Assume that the average temperature of the lake is 285K.
- Calculate the concentration of the insecticide on June 1st of the next year.
- How long will it take for the concentration of the insecticide to drop to 3.0 x 10-7 gcm-3?
(e)Iodide reduce iron (III) to iron (II) according to the reaction;
2Fe3+(aq) +2I-(aq) → 2Fe2+(aq) + I2(aq)
Experimentally, it was found that doubling the concentration of Fe3+, ions, doubled the rate of reaction. On the other hand, doubling the concentration of iodide ions increased the rate of the reaction by a factor of 4. Determine the order of reaction with respect to each reactant, the overall order and write the rate law of the reaction.
- (a)(i)Give any three (3) applications of common ion effect
(ii)The solubility of Pb(OH)2 in water is 6.7 x 10-6. Calculate the solubility of Pb(OH)2 in a buffer solution of Ph=8
(b)Hair bleaching solutions contain hydrogen peroxide, H2O2. The amount of hydrogen peroxide in the solution can be determined by making H2O2+ react with an acidic solution of potassium dichromate. The unbalanced equation for the reaction is;
H2O2(aq) + Cr2O72-(aq) + H+(aq) → O2(g) + Cr3+(aq) + H2O
A.30.00g sample of the bleach solution needed 75.8ml of 0.388MK2Cr2O7 to react completely with the bleach. Assuming no other compounds that react with K2Cr2O7 are in the solution, what is the mass percent of H2O2 in the bleach?
(c)For the titration of 75.00ml of 0.1M acetic acid with 0.1M NaOH, calculate the pH;
- Before the addition of any NaOH solution,
- After 25.00Ml of the base has been added,
- After half of the HC2H3O2 has been neutralized, and
- At the equivalent point
(d)When edta(ethylenediaminetetraacetic acid) is titrated against calcium salts, experiments show that one mole of edta reacts with one mole of Ca2+. This technique is used in clinical analysis to estimate the concentration of calcium in blood serum. Concentration between 7 and 13mg of calcium per 100cm3 of blood serum are normal.
One cubic centimeter of blood serum was taken from a patient and found to require 2.75cm3 of 0.0015M edta solution.
- Calcium the concentration of calcium in the patient’s blood.
- Is there an abnormal level of calcium concentration in the patient’s blood?
- (a)Consider the following ions; Cl-, K+, S2-, Sc3+ and Ca2+
Arrange them in order of;
- Increasing ionic size
- Increasing polarizing power
- Increasing polarisability
(b)Observe the isomers [Co(NH3)5Br] SO4 and [Co (NH3)5SO4] Br, then answer the questions that follows:
- Name the isomers
- What ions will the isomers yield in solution?
- Give two chemical tests that would be used to distinguish between the isomers
- What is the oxidation state and coordination number of cobalt in the complexes?
(c)50cm3 of a solution of 0.1M[Co(NH3)5Br]SO4 was mixed with 50cm3 of a solution of 0.1MKBr and the solution was made up to 200cm3. What is the concentration of Br in this solution? (Assume the two compounds ionize completely and supply two ions in solution)
(d) Explain the following observations;
- Lithium salts have a greater degree of covalent character than the other halides of the group.
- Li+ is far smaller than the metal ions but it moves through a solution less rapidly than the others.
- It is not possible to prepare hydrogen iodide by the action of concentrated sulphuric acid on potassium iodide
- AlCl3. 6H2O is acidic in aqueous solution while CH3COONa is basic in aqueous solution
- When Sulphur dioxide is bubbled through an acidified aqueous solution of K2Cr2O7, the yellow colour of the latter turns green.
- (a)Identify compounds A, B, C, D and reagents X, Y and Z in the following scheme of reactions
(b)Given the structure of compound K
Write down the chemical equation for the reaction between compound K and
- Bromine water
- feCl3
- Explain whether the compound will give iodoform test. If it gives positive iodofrm test, what are the reagents and condition for the test?
(c) The following experiment was carried out by a form six student.
A 9.2g sample of ethanol and 20.4g of 2-methylbutanoic acid were mixed in a flask and 2.0g of concentrated sulphuric acid was added. The mixture was refluxed for four hours and then fractionally distilled to give 17.4g of the crude ester. The ester was washed repeatedly with aqueous sodium carbonate until there was no more effervescence. After further washing with distilled water and drying, 15.6g of pure ester were obtained.
By referring to the experimental procedure above,
- Explain why the crude ester was washed repeatedly with aqueous sodium carbonate.
- State which gas was responsible for the effervesce
- Calculate how many moles of each reactant were reacted.
- Use your answer in (c)(iii) to calculate the percentage yield of pure ester obtained in the above experiment.
(d)Suggest the simple chemical test that would be used to distinguish the following compounds:
- Ethanol and acetone (Propanone)
- Ethanamine and N-methylethanamine
- (a)Name the following compounds according to the IUPAC system;
(b)An organic compound A(C5H10O) containing carbonyl group was reduced with Li[AlH4] to give compound B.B was dehydrated with concentrated sulphuric acid to give product C of molecular formula C5H6O. D gives positive iodoform reaction.
- Identify A, B, C and D compounds. Give their structural formula and IUPAC names.
- Give equations for all reaction
(c)Show how the following concersion may be achieved:
(d)Nylon 6-6 has a structure containing the following repeat unit;
[CO-(CH2)4-CONH-(CH2)6-NH]-
- Give the structures of the monomers from which this polymer could be made.
- What-type of polymer is this?
(e)Write the polymer section unit of
- Polystyrene
- Dacron
- Protein
FORM SIX CHEMISTRY EXAM SERIES 34
FORM SIX CHEMISTRY EXAM SERIES 34
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
KILIMANJARO REGIONAL COMMISSIONER’S OFFICE
FORM SIX MOCK EXAMINATION
CHEMISTRY 1
INSTRUCTIONS
- This paper consists of ten (10) questions in sections A, and B
- Answer all questions in section A and choose any two (2) questions from section B.
- Each question carries ten (10) marks in section A and fifteen (15) marks in section B
- Cellular phones and any other unauthorized materials are not allowed in the examination room
- Write your Examination Number on every page of your answer booklet(s)
- The following constant may be used.
- Gas constant, R=0.0821 atmLmol-1K-1 or 8.314JMol-1K-1,
- Rydberg’s constant, RH = atmLmol-1K-1 or 8.314JMol-1K-1,
- Velocity of light, c=3 x 108m/s
- Planck’s constant, h= 6.63 x 10-34Js
- Atomic masses: H= 1, C=12, N=14. O=16, P=31, Br=80
SECTION A (70 Marks)
Answer all questions in this section
- (a)Earlier we met the idea that the probability of finding the electron at any particular points is zero. We also spoke about the probability of finding the electron at a given distance from the nucleus. Try to explain why these two ideas are not contradictions of one another.
(b)Werner Heisenberg derived a remarkable result using a different (but compatible) theory to that of Schrodinger. He said that it was impossible to measure with complete accuracy both the position and momentum of an electron. This statement has come to be known as Heisenberg’s uncertainty principle. We can write the uncertainty in position ![]() x and momentum as
x and momentum as ![]() p. Heisenberg’s equation is;
p. Heisenberg’s equation is; ![]()
- If the position of an electron is known to within 10-12m, what is the uncertainty in its momentum?
- In our large-scale world, is there a limit (in principle) on the accuracy with which we can measure the momentum and position of a particle, e.g. a golf ball?
- Guess the effective value of Planck’s constant in the large-scale world.
(c)Why do the spacing between lines in hydrogen spectrum decreases as one goes away from the nucleus?
(d)An electron moves from n= 5 to a lower energy level in visible part of electromagnetic spectrum. Calculate:
- Energy possessed by electron in the new energy level (shell)
- Energy emitted during transition from n=5 to a lower energy level
- Frequency of radiation emitted.
- (a)Write short notes on the following;
- Hydrogen bonding
- Van der Waal forces
(b) Explain the following observations:
- AlCl3 is covalent while AlF3 is ionic.
- CO2 is non-polar molecule while SO2 is polar
(c)Using VSEPR theory, predict the molecular geometry and the type of hybridization in which the following molecules undergo;
(i) H2O (ii) SO3 (iii) CS2 (iv) SO42-
- (a)A bromide of phosphorus is found to diffuse in the gaseous state more slowly by a factor of 3.923 than nitrogen gas under the same conditions.
- Calculate the relative molecular mass of the bromide
- Given that bromide molecule contains one atom of phosphorus, write down its formulate
- (a)Explain the following
- Why is the osmotic pressure measurement preferred for determining molecular mass of protein?
- Why the equimolar solutions of sodium chloride and glucose are not isotonic?
(b)Arrange the following solutions in order of increasing of their boiling points;
0.1MKCl, 0.01M glucose, 0.01M MgCl2, 0.01MKCl.
(c)A 0.004kg of acetic acid (CH3COOH) is added to 500cm3 of water. If 25% of the acid is dissociated, what will be the depression in freezing point?
(Kf for water = 1.86° Ckg/Mol, density of water = 0.997/cm3).
- (a)With examples state the following;
- Basic oxides
- Acidic oxides
- Amphoteric oxides
(b)You are given a list of metal carbonates in the below. Tabulate your results by using (V) to indicate soluble carbonate and (X) for insoluble carbonate in the space provided.
| Metal carbonate | MgCO3 | (NH4)2CO3 | Na2CO3 | CuCO3 | PbCO3 | ZnCO3 |
| Solubility |
(c)Using a balanced chemical equation, describe the decomposition of the following compounds when heated.
- Sodium carbonate (Na2CO3)
- Hydrated crystals of aluminium chloride (AlCl3.6H2O(s))
- Anhydrous copper (II) sulphate (CuSO4(s))
- Sodium hydrogen carbonate (NaHCO3(s))
- (a)State and give examples for each of the following terms;
- Physical equilibrium
- Chemical equilibrium
(b)1dm3of dinitrogen tetraoxide, N2O4 weighs 2.5g at 60°C and 1 atm pressure. Find;
- The degree of dissociation of N2O4 into NO2
- The value of Kp
(c)When a yellow solution of iron (III) chloride, FeCl3 and a colourless solution of potassium thiocyanate, KSCN were mixed in a test tube, a red colour appeared and the following equilibrium was established;
Fe3+(aq) + SCN(aq) ![]() [Fe(SCN)]2+(aq)
[Fe(SCN)]2+(aq)
Yellow Red
- What is the effect on the Fe3+ ion concentration up adding KSCN to the equilibrium mixture?
- Why has a change in pressure no effect on equilibrium?
- The red colour faded when the test tube containing the equilibrium mixture was placed in an ice-water bath. State whether the value of Kc for this reaction is high or low at the lower temperature. Is the forward reaction exothermic or endothermic? Justify your answer.
- (a)(i)State Hess’s law of constant heat summation.
(ii)Use Hess’s law to calculate the enthalpy change in the hypothetical reaction
2MgCl(s) → Mg(s) + MgCl2(s). If the standard enthalpy of formation of MgCl is -130kJ/mol and the standard enthalpy of formation of MgCl2 is -640kJ/mol.
(b)The table below lists a number of some mean bond enthalpies,
| Bond | Mean bond enthalpy (kJj/mol) |
| C-C | 348 |
| C=C | 612 |
| C-H | 412 |
| O-H | 463 |
- Given that the enthalpy of combustion to form carbon dioxide and steam is
-2102Kj/mol for propane and -1977Kj/mol for propene. Determine the enthalpy for the oxidation of 1 mole of propane to propene and steam.
C3H8(g) + ½O2(g) → C3H6(g) + H2O(g)
- Use the mean bond enthalpies in the table above together with your answer in ((i) above to calculate the bond enthalpy of the O=O bond in the oxygen molecule.
(c)Calculate the heat of formation of anhydrous sodium chloride from;
2Na(s) + 2H2O(l) + → 2NaOHaq + H2(g) + H2(g) ![]() H=-87.0 kcals
H=-87.0 kcals
H2(g) + Cl2(g) + aq. 2HClaq → ![]() H=-78.60kcals
H=-78.60kcals
NaOHaq. + HClaq → NaClaq. + H2O(i)![]() H=-13.70kcals
H=-13.70kcals
NaCl(s) + aq.NaClaq. → ![]() H=+1.20 kcals
H=+1.20 kcals
2H2(g) + O2(g)2H2O(l) →![]() H=-136.0kcals
H=-136.0kcals
SECTION B (30 Marks)
Answer any two (2) questions from this section
- (a)Give the IUPAC names of the following compounds;
(b)When 20cm3 of a gaseous of a gaseous hydrocarbon A, are exploded with 150cm3 of oxygen, the residual gases occupied 110cm3. After shaking the residual gases with aqueous sodium hydroxide, the final volume was 30cm3 (all volumes were measured at the same temperature and pressure. What is the molecular formula of A.
(c)Outline how the following conversions can be achieved in not more than three steps.
- CH3CH2Cl → CH3CH2 – CH –CH2
| |
Cl Cl
- CH3CH2CH3 → CH3CHCH3
|
Br
(d) What mass of propene is obtained is from 34.0g of 1-iodopropane on heating with ethanoic KOH, if the yield is 36%
- (a)How does straight fertilizer differs from complete fertilizer. Give one example in each case.
(b)Give the name of the fertilizer whether is straight, mixed or complete fertilizer and the type of the nutrients which contain in the following fertilizer coding below:
(i) 10:20:15 (ii) 40:0:0 (iii) 40:8:0
(c)The soil of Mawenzi farm contains the following nutrients where all the values are given inmeq per 100g of oven dry soil: Ca2+=35, Mg2+=20, Na+= 4, H+=7, Al3+=10.
Calculate;
- Cation exchange capacity
- Percentage base saturation
- The quantity of Mg2+ in grams present in 100g of the dry soil
(d)A farm required 240kg of nitrogen. What weight of urea fertilizer coded 40:0:0 a farmer needs to add to his/her farm to meet the demand? Show clearly how you obtain your answer.
- (a)Primary alkyl halide A C4H9Br reacted with alcoholic KOH to give compound B. Compound B is reacted with HBr to give C which is an isomer of A. When C was reacted with sodium metal it give a compound D C8H18 that was different than the compound when n-butyl bromide was reacted with sodium with sodium. Give the structural formula of Compounds A to D
(b)Suggest suitable tests to distinguish the following compounds.
- Cyclopentane and Pent-2 ene
- Cyclopentanol and phenol
(c)Briefly explain why (CH3)3C-Cl reacts by SNl mechanism while CH3CH2CH2-Cl reacts by SN2 mechanism
(d)How does benzene react with the following?
- Chloroethane in the presence of AlCl3
- Chlorine in the presence of FeCl3
- Carbon monoxide in the presence of AlCl3
- Concentrated sulphuric acid and heat
- Chloroform in the presence of AlCl3
FORM SIX CHEMISTRY EXAM SERIES 33
FORM SIX CHEMISTRY EXAM SERIES 33
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
MOCK EXAMINATION SOUTHERN ZONE
(MTWARA AND LINDI)
132/3B CHEMISTRY 3B
(For Both School and Private Candidates)
(ACTUAL PRACTICAL B)
TIME: 3:20 HOURS Friday, 11thFebruary, 2022 a.m.
![]()
Instructions
1. This paper consists of Three (3) questions.
2. Answer all questions.
3. Question one (1) caries (20 marks), questions two (2) and three (3) each caries (15 marks).
4. Mathematical tables and Non programmable calculator may be used.
5. Write your Examination Number on every page of your answer booklet(s).
6. For your calculations the following constants may be useful:
H=1, C=12, O=16, Cl=35.5, S=32, Na=23, Cu=64, K=39, Fe=56.
Universal gas constant, R=0.0821 Atm dm3mol−1K−1 or 8.314Jmol−1K−1
GMV= 22.4dm3
Specific heat capacity of solution= 4.2Jg−1K−1
Density of solution= 1g/cm3
1. You are provided with the following:
X: A solution containing a mixture of sodium carbonate and sodium hydroxide in aqueous solution
Y: A solution containing 9.125g of pure hydrochloric acid in 0.25dm3 of equations solution.
Methyl orange indicator
Phenolphthalein indicator
Theory:
Reaction of hydrochloric acid with both sodium carbonate and sodium hydroxide can be represented by the following equations:
NaOH+HCl →NaCl+H2O
Na2CO3+HCl →NaHCO3+NaCl
NaHCO3+HCl →NaCl+H2O+CO2
Procedures:
(i) Pipette 20cm3 or 25cm3 of solution X into a conical flask.
(ii) Add two or three drops of P.O.P.
(iii) Titrate this solution against solution Y until color change is observed.
(iv) Record the first titre value.
(v) Add two drops of M.O to the same solution.
(vi) Continue to titrate until a second color change is observed.
(vii) Record the second titre value.
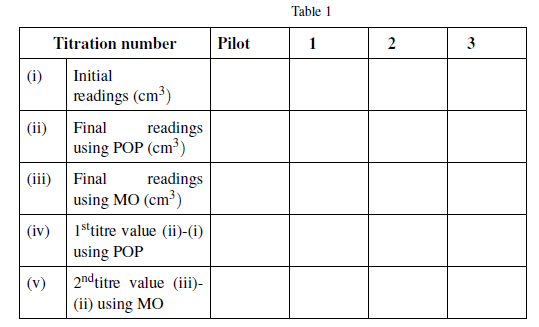
(viii) Repeat your titration procedure (i) to (vii) above two times and record your results as shown here below.
Results:
The volume of the pipette used was ![]() cm3
cm3
Burette readings
Table 1
| Titration number | Pilot | 1 | 2 | 3 | |
| (i) | Initial readings (cm3) | ||||
| (ii) | Final readings using POP (cm3) | ||||
| (iii) | Final readings using MO (cm3) | ||||
| (iv) | 1sttitre value (ii)-(i) using POP | ||||
| (v) | 2ndtitre value (iii)- (ii) using MO | ||||
Summary:
.............cm3 of solution X required..... cm3 of Y when POP was the indicator .............cm3 when MO was the indicator.
Questions:

(b) Indicate the chemical equations which were appropriate to the titre value found when using.
(i) P.O.P
(ii) M.O
(c) Calculate the concentration of.
(i) Sodium hydroxide in mol/dm3 in solution X
(ii) Sodium carbonate in mol/dm3 in solution X
(d) Calculate the percentage composition by mass of both sodium hydroxide and sodium carbonate in solution X.
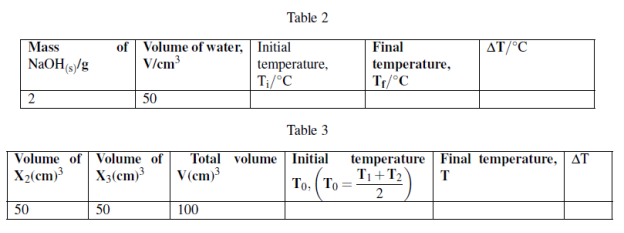
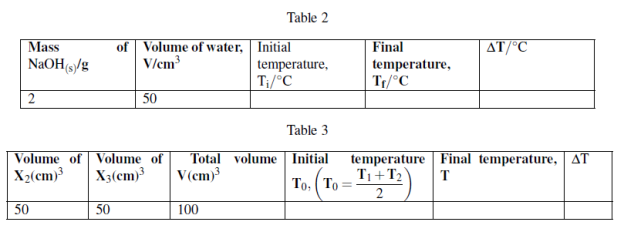
You are provided with the following:
X1: 2g of NaOH pellets.
X2: 1M sodium hydroxide solution. X3: 1M Hydrochloric acid solution.
X4: Distilled water.
Procedures 1:
(i) Measure 50cm3 of X4 in 100cm3 plastic beaker or any 100cm3 plastic container.
(ii) using thermometer to measure the initial temperature of water, T1 = ![]() .
.
(iii) Take 2g of sodium hydroxide pellets and dissolve in 50cm3 water in a plastic beaker stir the solution and make sure all the solute dissolve. Record the final temperature of solution when no further temperature change.
(iv) Tabulate the results as follows:
Procedures 2:
(i) Measure 50cm3 of X2 and put in 100cm3 plastic beaker. Record the initial temperature, T1
(ii) Measure 50cm3 of X3 in measuring cylinder and measure its initial temperature, T2 while in measuring cylinder.
(iii) Pour X3 solution from measuring cylinder to plastic beaker containing X2, stir well to mix then measure the final temperature attained using the thermometer.
(iv) Tabulate the results as follows:
Questions:
FORM SIX CHEMISTRY EXAM SERIES 28
FORM SIX CHEMISTRY EXAM SERIES 28
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
MOCK EXAMINATION SOUTHERN ZONE (MTWARA AND LINDI)
132/3A CHEMISTRY 3A
(For Both School and Private Candidates)
(ACTUAL PRACTICAL A)
TIME: 3:20 HOURS Tuesday, 08th February, 2022 a.m.
![]()
Instructions
1. This paper consists of Three (3) questions.
2. Answer all questions.
3. Question one (1) caries (20 marks), questions two (2) and three (3) each caries (15 marks).
4. Mathematical tables and Non programable calculator may be used.
5. Write your Examination Number on every page of your answer booklet(s).
6. For your calculations the following constants may be useful:
H=1, C=12, O=16, Cl=35.5, S=32, Na=23, Cu=64, K=39, Fe=56.
Universal gas constant, R=0.0821 Atm dm3mol−1K−1 or 8.314Jmol−1K−1
GMV= 22.4dm3
Specific heat capacity of solution= 4.2Jg−1K−1
Density of solution= 1g/cm3
1. You are provided with the following:
X: A solution made by dissolving 0.79g of KMnO4 in 250cm3 of solution
Y: A solution made by dissolving 13.90g of FeSO4.XH2O in 500cm3 of aqueous solution acidified with sulphuric acid..
Z: A dilute sulphuric acid Procedures:
(i) Put X in the burette.
(ii) Pipette 20 or 25cm3 of Y into conical flask. Add 20cm3 of Z to the same conical flask.
(iii) Titrate the solution in conical flask against X in the burette until permanent pink colour just appear. Record the burette reading.
(iv) Repeat procedure (i) to (iii) three times and tabulate your results as shown below.
Results:
The volume of the pipette used was......................cm3
The volume of the burette used was .......................cm3
Burette readings
| Titration Number | Pilot | 1 | 2 | 3 |
| Initial volume (cm3) | ||||
| Final volume (cm3) | ||||
| Volume used (cm3) |
Summary:
.............................. cm3 of Y required ................. cm3 of X for complete reaction.
Questions:
(a) (i) Write the redox half reactions for this titration.
(ii) Write the overall balanced ionic redox reaction.
(iii) Indicate the species which is oxidant and the one which is reductant.
(b) Explain why an indicator is not used in this kind of experiment.
(c) Explain why sulphuric acid and not hydrochloric acid is used in a particular experiment.
(d) Calculate:
(i) Concentration of FeSO4.XH2O in g/dm3
(ii)Concentration of KMnO4 in g/dm3
(iii) Molarity of KMnO4
(iv) Molarity of FeSO4
(v) Molar mass of FeSO4.XH2O (vi) Value of X in the formula FeSO4.XH2O
2. You are provided with the following:
K: 4g of hydrated sodium carbonate (Na2CO3.10H2O)
J: 2g of anhydrous sodium carbonate Na2CO3
Thermometer, small plastic beaker and distilled water.
Procedures:
(i) Measure 50cm3 of distilled water and put in small plastic beaker. Record the initial temperature, T1 = .
(ii) Dissolve 4g of Na2CO3.10H2O in the small plastic beaker contain water while stirring record the final temperature of solution, T2 = ................
(iii) Discard the contents in the plastic beaker and wash it then measure 50cm3 of distilled water and put in the plastic beaker. As usual take the initial temperature of water using the thermometer, T3 = ..............
(iv) Take 2g of anhydrous sodium carbonate, dissolve in plastic beaker containing distilled water, while stirring record the final temperature, T4 = ...................
Results:
| EXPERIMENT | INITIAL TEMPERATURE | FINAL TEMPERATURE | TEMPERATURE CHANGE |
| 1 | |||
| 2 |
Questions:
(i) Calculate the heat change in each experiment.
(ii) Calculate the molar heat change in each experiment.
(iii) Calculate the heat of hydration of anhydrous sodium carbonate to hydrated sodium carbonate
(iv) Comment on the different in the molar heat change of solution for salt K and
L
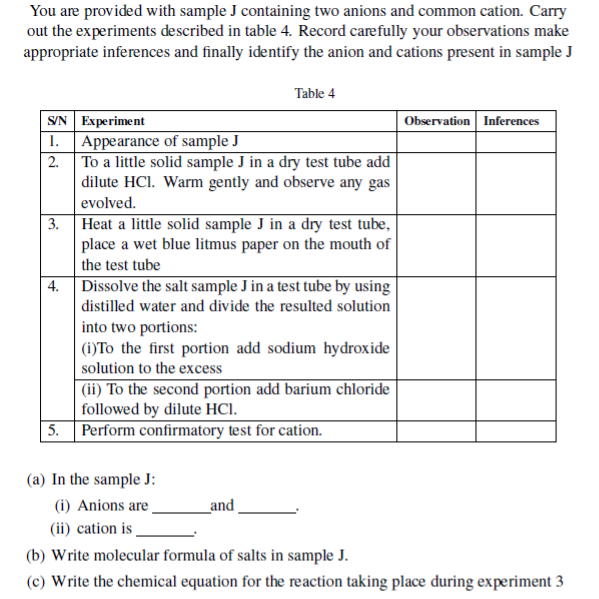
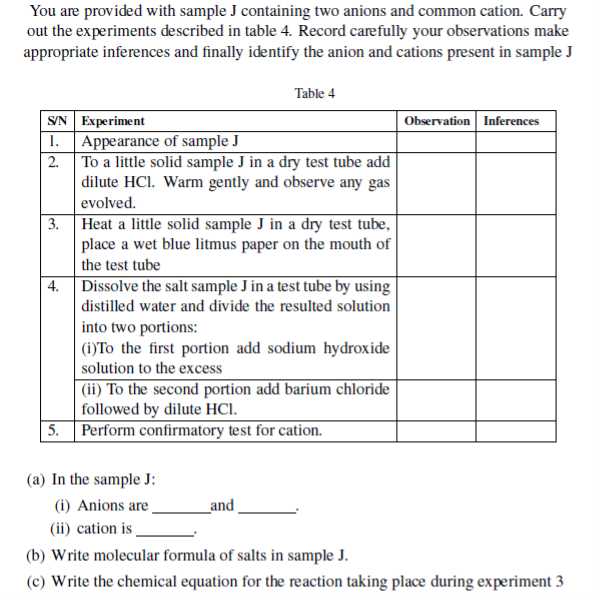
3. Sample Q contains a two cations and two anions. Use the information given in the experiment column to complete the observations and inferences and hence identify the two cations and the two anions
| S/N | Experiment | Observation | Inferences |
| 1. | Take a spatulaful of sample Q into a boiling tube and add about 3cm3 of distilled water. Heat gently the mixture for a bout one minute while swirling the test-tube. Filter to obtain a clear solution and divide a resulting solution into three portions. (a)To first portion add NaOH solution | ||
| (b)To second potion add NH4OH solution. | |||
| (c) To the second portion add dilute HNO3 acid followed by AgNO3 solution and then NH3 solution. | |||
| 2. | (a) Dissolve a residue in little quantity of Conc.HCl and identify the gas evolved | ||
| (b) Dilute the resulting solution in (a) above with water and divide into two portions (i) To first portion add dilute magnesium sulphate solution. | |||
| (ii) To second portion add potassium iodide solution |
Calculation
(a) The cations in sample Q are .
(b) The anions in sample Q are
(c) Sample Q is a mixture of
(d) Give one application of cation obtained in life.
FORM SIX CHEMISTRY EXAM SERIES 27
FORM SIX CHEMISTRY EXAM SERIES 27
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
MOCK EXAMINATION SOUTHERN ZONE
(MTWARA AND LINDI)
132/3B CHEMISTRY 3B
(For Both School and Private Candidates)
(ACTUAL PRACTICAL B)
TIME: 3:20 HOURS Friday, 11thFebruary, 2022 a.m.
![]()
Instructions
1. This paper consists of Three (3) questions.
2. Answer all questions.
3. Question one (1) caries (20 marks), questions two (2) and three (3) each caries (15 marks).
4. Mathematical tables and Non programmable calculator may be used.
5. Write your Examination Number on every page of your answer booklet(s).
6. For your calculations the following constants may be useful:
H=1, C=12, O=16, Cl=35.5, S=32, Na=23, Cu=64, K=39, Fe=56.
Universal gas constant, R=0.0821 Atm dm3mol−1K−1 or 8.314Jmol−1K−1
GMV= 22.4dm3
Specific heat capacity of solution= 4.2Jg−1K−1
Density of solution= 1g/cm3
1. You are provided with the following:
X: A solution containing a mixture of sodium carbonate and sodium hydroxide in aqueous solution
Y: A solution containing 9.125g of pure hydrochloric acid in 0.25dm3 of equations solution.
Methyl orange indicator
Phenolphthalein indicator
Theory:
Reaction of hydrochloric acid with both sodium carbonate and sodium hydroxide can be represented by the following equations:
NaOH+HCl →NaCl+H2O
Na2CO3+HCl →NaHCO3+NaCl
NaHCO3+HCl →NaCl+H2O+CO2
Procedures:
(i) Pipette 20cm3 or 25cm3 of solution X into a conical flask.
(ii) Add two or three drops of P.O.P.
(iii) Titrate this solution against solution Y until color change is observed.
(iv) Record the first titre value.
(v) Add two drops of M.O to the same solution.
(vi) Continue to titrate until a second color change is observed.
(vii) Record the second titre value.
(viii) Repeat your titration procedure (i) to (vii) above two times and record your results as shown here below.
Results:
The volume of the pipette used was ![]() cm3
cm3
Burette readings
Summary:
.............cm3 of solution X required..... cm3 of Y when POP was the indicator .............cm3 when MO was the indicator.
Questions:

(b) Indicate the chemical equations which were appropriate to the titre value found when using.
(i) P.O.P
(ii) M.O
(c) Calculate the concentration of.
(i) Sodium hydroxide in mol/dm3 in solution X
(ii) Sodium carbonate in mol/dm3 in solution X
(d) Calculate the percentage composition by mass of both sodium hydroxide and sodium carbonate in solution X.
You are provided with the following:
X1: 2g of NaOH pellets.
X2: 1M sodium hydroxide solution. X3: 1M Hydrochloric acid solution.
X4: Distilled water.
Procedures 1:
(i) Measure 50cm3 of X4 in 100cm3 plastic beaker or any 100cm3 plastic container.
(ii) using thermometer to measure the initial temperature of water, T1 = ![]() .
.
(iii) Take 2g of sodium hydroxide pellets and dissolve in 50cm3 water in a plastic beaker stir the solution and make sure all the solute dissolve. Record the final temperature of solution when no further temperature change.
(iv) Tabulate the results as follows:
Procedures 2:
(i) Measure 50cm3 of X2 and put in 100cm3 plastic beaker. Record the initial temperature, T1
(ii) Measure 50cm3 of X3 in measuring cylinder and measure its initial temperature, T2 while in measuring cylinder.
(iii) Pour X3 solution from measuring cylinder to plastic beaker containing X2, stir well to mix then measure the final temperature attained using the thermometer.
(iv) Tabulate the results as follows:
Questions:
FORM SIX CHEMISTRY EXAM SERIES 26
FORM SIX CHEMISTRY EXAM SERIES 26
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
MOCK EXAMINATION SOUTHERN ZONE (MTWARA AND LINDI)
132/3A CHEMISTRY 3A
(For Both School and Private Candidates)
(ACTUAL PRACTICAL A)
TIME: 3:20 HOURS Tuesday, 08th February, 2022 a.m.
![]()
Instructions
1. This paper consists of Three (3) questions.
2. Answer all questions.
3. Question one (1) caries (20 marks), questions two (2) and three (3) each caries (15 marks).
4. Mathematical tables and Non programable calculator may be used.
5. Write your Examination Number on every page of your answer booklet(s).
6. For your calculations the following constants may be useful:
H=1, C=12, O=16, Cl=35.5, S=32, Na=23, Cu=64, K=39, Fe=56.
Universal gas constant, R=0.0821 Atm dm3mol−1K−1 or 8.314Jmol−1K−1
GMV= 22.4dm3
Specific heat capacity of solution= 4.2Jg−1K−1
Density of solution= 1g/cm3
1. You are provided with the following:
X: A solution made by dissolving 0.79g of KMnO4 in 250cm3 of solution
Y: A solution made by dissolving 13.90g of FeSO4.XH2O in 500cm3 of aqueous solution acidified with sulphuric acid..
Z: A dilute sulphuric acid Procedures:
(i) Put X in the burette.
(ii) Pipette 20 or 25cm3 of Y into conical flask. Add 20cm3 of Z to the same conical flask.
(iii) Titrate the solution in conical flask against X in the burette until permanent pink colour just appear. Record the burette reading.
(iv) Repeat procedure (i) to (iii) three times and tabulate your results as shown below.
Results:
The volume of the pipette used was......................cm3
The volume of the burette used was .......................cm3
Burette readings
| Titration Number | Pilot | 1 | 2 | 3 |
| Initial volume (cm3) |
|
|
|
|
| Final volume (cm3) |
|
|
|
|
| Volume used (cm3) |
|
|
|
|
Summary:
.............................. cm3 of Y required ................. cm3 of X for complete reaction.
Questions:
(a) (i) Write the redox half reactions for this titration.
(ii) Write the overall balanced ionic redox reaction.
(iii) Indicate the species which is oxidant and the one which is reductant.
(b) Explain why an indicator is not used in this kind of experiment.
(c) Explain why sulphuric acid and not hydrochloric acid is used in a particular experiment.
(d) Calculate:
(i) Concentration of FeSO4.XH2O in g/dm3
(ii)Concentration of KMnO4 in g/dm3
(iii) Molarity of KMnO4
(iv) Molarity of FeSO4
(v) Molar mass of FeSO4.XH2O (vi) Value of X in the formula FeSO4.XH2O
2. You are provided with the following:
K: 4g of hydrated sodium carbonate (Na2CO3.10H2O)
J: 2g of anhydrous sodium carbonate Na2CO3
Thermometer, small plastic beaker and distilled water.
Procedures:
(i) Measure 50cm3 of distilled water and put in small plastic beaker. Record the initial temperature, T1 = .
(ii) Dissolve 4g of Na2CO3.10H2O in the small plastic beaker contain water while stirring record the final temperature of solution, T2 = ................
(iii) Discard the contents in the plastic beaker and wash it then measure 50cm3 of distilled water and put in the plastic beaker. As usual take the initial temperature of water using the thermometer, T3 = ..............
(iv) Take 2g of anhydrous sodium carbonate, dissolve in plastic beaker containing distilled water, while stirring record the final temperature, T4 = ...................
Results:
| EXPERIMENT | INITIAL TEMPERATURE | FINAL TEMPERATURE | TEMPERATURE CHANGE |
| 1 |
|
|
|
| 2 |
|
|
|
Questions:
(i) Calculate the heat change in each experiment.
(ii) Calculate the molar heat change in each experiment.
(iii) Calculate the heat of hydration of anhydrous sodium carbonate to hydrated sodium carbonate
(iv) Comment on the different in the molar heat change of solution for salt K and
L
3. Sample Q contains a two cations and two anions. Use the information given in the experiment column to complete the observations and inferences and hence identify the two cations and the two anions
| S/N | Experiment | Observation | Inferences |
| 1. | Take a spatulaful of sample Q into a boiling tube and add about 3cm3 of distilled water. Heat gently the mixture for a bout one minute while swirling the test-tube. Filter to obtain a clear solution and divide a resulting solution into three portions. (a)To first portion add NaOH solution |
|
|
| (b)To second potion add NH4OH solution. |
|
| |
| (c) To the second portion add dilute HNO3 acid followed by AgNO3 solution and then NH3 solution. |
|
| |
| 2. | (a) Dissolve a residue in little quantity of Conc.HCl and identify the gas evolved |
|
|
| (b) Dilute the resulting solution in (a) above with water and divide into two portions (i) To first portion add dilute magnesium sulphate solution. |
|
| |
| (ii) To second portion add potassium iodide solution |
|
|
Calculation
(a) The cations in sample Q are .
(b) The anions in sample Q are
(c) Sample Q is a mixture of
(d) Give one application of cation obtained in life.
FORM SIX CHEMISTRY EXAM SERIES 25
FORM SIX CHEMISTRY EXAM SERIES 25
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
MOCK EXAMINATION SOUTHERN ZONE (MTWARA AND LINDI )
132/2CHEMISTRY 2
(For Both School and Private Candidates)
Time: 3 HoursFriday, 04thFebruary, 2022 p.m.
![]()
Instructions
1.This paper consists of six (6) questions.
2.Answer only five (5) questions.
3.Each question carries twenty (20) marks.
4.Mathematical tables and Non programable calculators may be used.
5.Cellular phones and any unauthorized materials are not allowed in the examination room.
6.Write your Examination Number on every page of your answer booklet(s).
7.For calculations you may use the following constants:
•Universal gas constant, R= 8.314JK−1mol−1or 0.0821K−1atm mol−1K−1dm3
•Standard temperature =273K
•Standard pressure =1atm =760mmHg =1.0×105Nm−2
•1 Faraday= 96500Cmol−1
•GMV=22.4dm3
•1 Litre=1dm3= 1000cm3
•Velocity of light,C=3.0×108m/s
•Atomic masses: Ag= 108, Cl= 35.5, Mn= 55, Fe= 56, I= 127
1.(a) (i) What is ideal solution.(01 mark)
(ii)Ideal solution differs from non-ideal solution by four areas, by using four points give factors contributes to ideal solution formation. (04 marks)
(iii)By using well labelled diagram show that Acetone and Ethanol mixture form a non-ideal solutions.(05 marks)
(b)When 0.7dm3of an aqueous solution containing 6g of a solute B per litre was shaken with 200cm3of heptanol, 3.21g of the solute B was extracted. Assuming the molecular state of the solute remained the same in both solvents, Calculate:
(i)Value of distribution coefficient.(03 mark)
(ii)Mass of the solute B which remained in the aqueous solution after a further shaking with 200cm3of heptanol.(03 marks)
(c)(i) What is steam distillation?.(01 mark)
(ii) Chlorobenzene which is insoluble in water is steam distilled at 98◦C and atmospheric pressure of 101300Pa. A sample of steam distillate contains 247g of chlorobenzene for every 16g of H2O. Calculate the vapour pressure of water and chlorobenzene at 98◦C. (03 marks)
2.(a) Define the following terms as applied in chemistry
(i)Electric double layer (01![]() marks)
marks)
(ii)Electrode potential(01![]() marks)
marks)
(iii)Salt Bridge.(01![]() marks)
marks)
(b)(i) Differentiate between Kohlrausch law and Ostwald dilution law.
(02 marks)
(ii)Give five applications of electrochemical series in life of chemistry.
21/2marks)
(iii)Show the relationship between weak electrolyte![]()
(c)(i) During supplying of food to students with silver tray it observed some silver particles were plated out from silver tray. How many grams of silver could be plated out on a serving tray by electrolysis of a solution silver +1 for a period of 8 hours at a passed current of 8.35amphere.
(04 marks)
(ii) What mass of NH4Cl must be added to 0.5L of 1molL−1NH3solution to yield a solution with a PH of 9.0. Assume that no change in volume with Kb of 1.8 x 10-5(03 marks)
(d)Give two components of buffer solution.(02 marks)
3.(a) Explain the following concepts:-
(i)Is there any reaction for which the rate does not change with the initial concentrations of the reactants?. (02 marks)
(ii)A lump of coal burns at a moderate rate in air while coal dust burns explosively.(02 marks)
(iii)Liquid bromide reacts slowly as compared to bromine vapour.(02 marks)
(iv)The energy of activation determines its rate.(02 marks)
(b)(i) What is half life of the reaction.(01 mark)
(ii) Consider the following reaction
![]()
If the rate constant K at 310◦C and 420◦C is 1.3×10−3dm−3mol−1s−1and 6.3×10−2dm−3mol−1s−1respectively, What is the activation energy Eafor the reaction?.(04 marks)
(c)The following table contains data which were collected at 298K for the reaction.
![]()
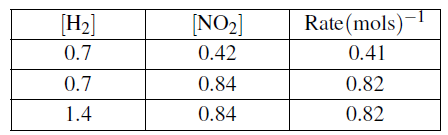
(i)Write down the rate expression for the reaction.(03 marks)
(ii)Calculate the order of the reaction with respect to each reactant and write the overall order of reaction. (04 marks)
4.(a) (i) Describe four features of coordination compounds.(04 marks)
(ii)Differentiate between charged complex from neutral complex. (02 marks)
(iii)How Ambidentante ligand differ from Monodentate ligand with example. (02 marks)
(b) Give explanations and equation when possible from the following observations.
(i)Sodium metal is very soft compared with magnesium metal.(01 mark)
(ii)Silicon tetrachloride (SiCl4) can be hydrolysed by water, but carbon tetrachloride (CCl4) cannot be hydrolysed. (01 mark) (iii) Aluminium metal does not react hot water or steam. (01 mark) (iv) F2and Cl2exists as gases, Br2exist in the liquid form and I2exist as solid.(01 mark)
(v) Lithium nitrate is decomposed thermally completely but potassium nitrate decomposed only partially.(01 mark)
(c) Briefly explain the extraction of aluminium from its principal ore, bauxite
(Al2O3.2H2O) by Bayer’s Method.(07 marks)
5.(a) (i) What is a nucleophilic addition reaction.(01![]() marks)
marks)
(ii)Describe the mechanism of nucleophilic addition reaction to a carbonyl compound.(03 marks)
(iii)Explain why ethanal are more reactive than acetone towards nucleophilic addition reaction.(02![]() marks)
marks)
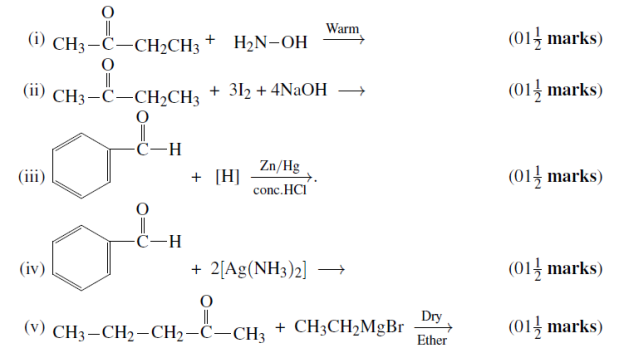
(b)Complete the following chemical reactions:
(c)An unknown ester E was found to have molecular formula C5H10O2. When E was hydrolysed with water in the presence of mineral acid, it produced carboxylic acid F with alcohol G. The treatment of G with SOCl2gave an alkyl chloride H. When H was treated with KCN, a product I was formed which on hydrolysis with water and acid produced carboxylic acid F.
(i)Use this information to deduce the structure and name of the original ester. (02 marks)
(ii)Identify F to I and write all chemical reactions involved.(06 marks)
6.(a) An organic compound C2H4O gives red precipitate when warmed with Fehling’s solution. It also undergo aldol condensation in the presence of NaOH.
(i)Write structure and IUPAC name of the compound.(01 mark)
(ii)What is the state of hybridization of the carbon atoms in the compound.
(01 mark)
(iii) Write all reactions involved in the compound.(02![]() marks)
marks)
(b) Show how the following conversion may be achieved.
(c) An ester A (C4H8O2), on treatment with excess methyl magnesium chloride followed by acidification, gives an alcohol B as the sole organic product. Alcohol B, on oxidation with NaOCl followed by acidification, gives acetic acid.
- Decide the structure of A and B
- Show all reactions involved
FORM SIX CHEMISTRY EXAM SERIES 4
FORM SIX CHEMISTRY EXAM SERIES 4
PRESIDENT’S OFFICE
REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT
ADVANCED CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
MOCK EXAMINATION SOUTHERN ZONE
(MTWARA AND LINDI)
132/1CHEMISTRY 1
(For Both School and Private Candidates)
Time: 3 HoursWednesday, 02ndFebruary, 2022 a.m.
![]()
Instructions
1.This paper consists of sections A and B with a total of ten(10) questions.
2.Answer ALL questions in section A and two(2) questions from section B.
3.Mathematical tables and non-programable calculators may be used.
4.Cellular phones and any unauthorized materials are not allowed in the examination room.
5.Write your Examination Number on every page of your answer booklet(s).
6.The following information may be used.
(a)Universal gas constant, R=8.314JK−1mol−1or 0.0821atmdm3mol−1.
(b)Rydberg constant RH= 1.09678×107m−1
(c)standard pressure =1 atm=760mmHg=1.0× 105N/m2
(d)1 Mass of electron= 9.11×10−31kg
(e)Density of water= 1g/cm3
(f)Velocity of light, C= 3.0×108m/s
(g)Avogadron’s constant =6.022×1023mol−1
(h)1A˚ = 1.0×10−10m
(i)Planks constant, h= 6.63×10−34Js
(e) Atomic masses
H=1, C=12, O=16, N=14, Cl=35.5, Br=80, Na=23.
This paper consists of six printed pages
SECTION A (70 MARKS)
1.(a) Differentiate
(i)Emission spectra and absorption spectra
(ii)Line spectra and continuous spectra
(iii)Atomic orbital and degenerate orbital(03 marks)
(b)What is the wavelength of a photon (in nanometers) emitted during a transition from the fifth to second energy levels in hydrogen atom? To what region of the spectrum does this wavelength correspond? (04 marks)
(c)Suppose that the uncertainty in determining the position of an electron circling an atom in an orbit is 0.4A˚. What is the uncertainty in the velocity? (03 marks)
2.(a) What do you understand by
(i)Octet rule
(ii)hybridization of atomic orbital
(iii)Orbital(03 marks)
(b)Using sketches, briefly explain three possible overlaps that can lead to formation of a sigma bond
(c)Give two reasons for the observed difference in bond strength between sigma and pi bonds in compounds.
(d)Predict the geometry of water, basing on the valence shell Electron pair Repulsion (VSEPR) theory.
3.(a) What do you understand by lattice enthalpy? What is its significance? (02 marks)
(b)Some values of lattice enthalpies/kJ/mol are NaCl-771, KCl-707, NaF-918, CsF-747, NaI-699, MgO-3791, BaO-3054. Comment on differences of lattice enthalpies of
(i)NaCl and KCl
(ii)NaCl and NaF
(iii)MgO and BaO
(iv)NaCl and MgO(04 marks)
(c)Construct a Born-Haber cycle, and use it to calculate the standard lattice energy of cadmium (II) iodide
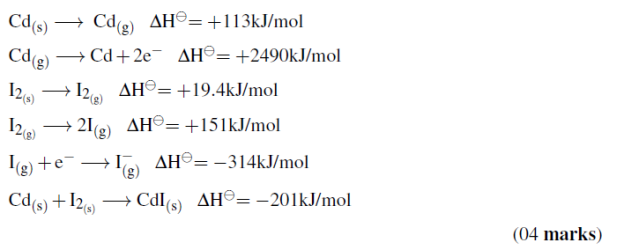
4.(a) (i) What is an ideal gas?
(ii) The pressure exerted by 12g of an ideal gas at temperature t0C in a vessel of volume V litres is one atmosphere. When the temperature is increases by 10%. Calculate the temperature t and volume V. Molecular mass of the gas = 120u(04![]() marks)
marks)
(b)Two flasks at the same temperature are joined by a glass tube with a stopcock. Flask A is a 4.0L flask containing N2(g)at 2.0 atm, while flask B is a 10.0L flask containing carbon monoxide gas at 1.4 atm. calculate the total pressure when the stopcock is opened. (02![]() marks)
marks)
(c)A straight glass tube has two inlets X and Y at the two ends. The length of the tube is 50cm. HCl gas through the inlet X and NH3gas through the inlet Y are allowed to enter the tube at the same time. White fumes first appear at a point P inside the tube. Find the distance of P from X (03 marks)
5.(a) The Oxo-acids have acidity strength in the order HClO4>H2SO4>H3PO4.
Explain the trend of acidity of these oxo-acids.(03 marks)
(b)With the aid of chemical equation (s) explain how you can prepare soluble chlorides.(04 marks)
(c)By the aid of chemical equations describe the following
(i)Basic oxides
(ii)Amphoteric oxides
(iii)Acidic oxides(03 marks)
6.(a) Explain the following
(i)Homogeneous catalyst
(ii)Heterogeneous equilibrium
(iii)Dynamic equilibrium(03 marks)
(b)Consider the following gas phase reaction equilibrium involving N2O4.
Reaction 1: N2O4(g)2NO2(g)k1= 0.115
Reaction 2: 2N2O4(g)2N2O(g)k2= 1.37×10−1
Determine the equilibrium constant of the reaction.
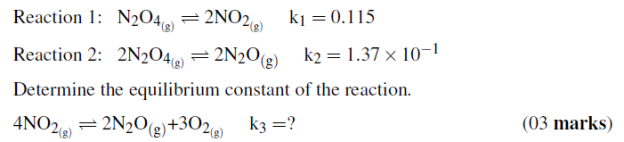
(c)At the start of a reaction, there are 0.249 mol N2, 3.21×10−2mol H2, and 6.42×10−4mol NH3in a 3.50L reaction vessel at 375◦C. If the equilibrium constant (kc) for the reaction N2(g)+3H2(g)→2NH3(g)is 1.2 at this temperature, decide whether the system is at equilibrium. If it is not, predict which way the net reaction will proceed to achieve equilibrium.(04 marks)
7.(a) Distinguish sigma bond from pi bond(03 marks)
(b)Predict the shapes of the following molecules using VSEPR theory
(i)AsF5
(ii)PCl3
(iii)SF6(03 marks)
(c)(i) Although both CO2and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2is linear. Explain this on basis of dipole moment.
(ii) Write two significance of dipole moment.(04 marks)
SECTION B (30 MARKS)
8.(a) Account for the following
(i)Benzene undergo addition reactions under harsh conditions.
(ii)Nucleophilicity of benzene is affected by the substituent attached to it
(iii)Halogens are deactivators but direct the coming electrophiles to ortho and para positions.(03 marks)
(b)Compound A, C7H14is treated with bromine in presence dichloromethane to give B, C7H14Br2. Compound B is treated with NaOH in presence of ethanol followed by NaNH2resulting in the formation of C. Compound C react with hydrogen in presence nickel catalyst to form 2-methlyhexane. Compound C have no reaction with ammoniacal silver nitrate. Ozonolysis of A gives aldehyde D and ethanal . Deduce the structure formula of A, B, C and D.
Write all the chemical reactions.(05 marks)
(c)Three hydrocarbons, D, E and F, all have the molecular formula C6H12. D decolourises an aqueous solution of bromine and shows geometric isomerism. E also decolourises an aqueous solution of bromine but does not show geometric isomerism. F does not decolourises an aqueous solution of bromine. Draw the structural formula of D, E and F. Write all the chemical reaction.(02![]() marks)
marks)
(d)How the following conversion can be achieved in not more than four steps.
(i)Butylchloride to But-1-yne(01![]() marks)
marks)
(ii)Ethene to benzene(01![]() marks)
marks)
(iii)But-1-ene to Butan-2-one.(01![]() marks)
marks)
9.(a) Explain the difference between the boiling temperatures of the following compounds:

(01![]() marks)
marks)
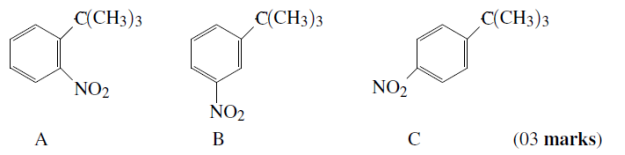
(b)Arrange the following products according to the percentage (%) yield obtained from the nitration of ter-butylbenzene. Justify the order.
(d)Give one chemical test to distinguish the following
(i)But-2-ene and butane
(ii)Benzyl chloride and chlorobenzene
(iii)1-chloropropane, 2-chloropropane and 2-chloro-2methylpropane
(04![]() marks)
marks)
10.(a) Define the following terms
(i)Liming
(ii)Cation exchange capacity
(iii)Percentage base saturation
(iv)Macronutrients(04 marks)
(b)A soil sample was collected from certain farm for laboratory analysis. After being analyzed the soil had the following exchangeable cations.
Ca2+= 30meq/100g
Mg2+= 16meq/100g
K+= 39meq/100g
H+= 4meq/100g
(b) How many milligrams of these elements were in the soil and what is the percentage base saturation of the soil sample. (07 marks)
(c) Mention four (4) factors of which influence the soil acidity? (02 marks)
(d) (i) What are the benefits of liming?(mention any four) (02 marks)
(ii) Not all calcium and magnesium compounds are suitable for liming.
Explain(02 marks)
FORM SIX CHEMISTRY EXAM SERIES 3
FORM SIX CHEMISTRY EXAM SERIES 3
PRESIDENT OFFICE
REGIONAL ADMINISTRATION AND LOCAGOVERNMENT
ILBORU SECONDARY SCHOOL
FORM VI PRE-NATIONAEXAMINATION
CHEMISTRY 1 SERIES 4
TIME 3:00HRS13th
SECTION A:
Answer all questions from this section
1.(a) Give three (3) main ideas of the Bohr atomic model
(b)Give three (3) shortcomings of the Bohr atomic mode
(c) i) Calculate the wavelength of a particlei.e 6.0 × 10-2kg tennis ball served at 63ms-1 ii) Calculate the uncertainty in the position of an electron if the uncertainty in its velocity is5.7 ×105 m/s 2.a) Why do NH3, H2O and HF have higher boiling point than those of PH3, H2S and HBr? b) Account for the following observations, although CO2 and SO2 have the same empirical formula, CO2 is non - polar while SO2 is a polar. (c) Discussthe necessaryconditions for theformationofionic andcovalent bonds. 3.(a) Define the following terms (i) Critical Pressure (ii)Critical Temperature (iii)Graham’s Law of Diffusion (iv) Boiling Point (v) Cryoscopic Constant (b) The mass of 243cm3 of a gas at 273K and at an atmospheric pressure of1 atm is 0.162g. 4.a)What do you understand by the following term (i)Heat of reaction (ii) Heat of combustion b)Carefully study the following information given below; (ii) Define the standard enthalpy change corresponding to∆H25 and ∆ H7 indicatedabove . (iii)Calculate∆H7 given the following values 5.Indicate any reaction involveseither the side chain or the aromatic ring or both of these which occur between phenylethene (i) Br2 (ii)Br2 (iii)H2 / Niat250C (iv) AcidifiedKMnO4(aq) (v) (v) H2 / Niat2000C 6.(a) Explain the following (i) A solution of MgCl2 freezes at a lower temp lower than that of glucose. (ii)It is notpossible to obtainMgCl2 from its aqueous salt byevaporation . (iii)SolidCO2 andSiO2 arerepresentedby similar empirical formula butdiffer in theirphysical properties . (b) Completeand balancethefollowingequations (c) What ismeant by thefollowin (i) Electron affinity (ii)Hydrogenbonding (iii)Electronegativity 7.(a) Briefly explain three limitations for distribution law. (b) A solution of 6g of substance X in 50mls of aqueous solution is in equilibrium at roomtemperature with a solution of X in diethyl ether containing 108g of X in 100ml. Calculate themass of x extracted by shaking 100ml of an aqueous solution containing 10g of X in, (i)100ml of ether (ii) 50ml of ether twice at room temperature. SECTION B: Answer two questions from this section’ 8.(a) Distinguish between the following (i)Phenol and alcohol (ii) Ethanoic acid and methanoic acid (iii) Ethanol and propanal (iv)Benzoic acid and benzene (b) A halo alkaneP(C5H11Br) reactswith aqueousSodiumhydroxideto give Q(C5H12O). Qreacts with concentratedH2SO45 9.(a) Dissociation of PCl5 decreases in presence of increase in Cl2. why? (b) Write the equilibrium constant for the followin c) The equilibrium constant Kc for A(g) d) In the equilibrium H22![]() H3 = + 19.4kJ/mol,
H3 = + 19.4kJ/mol, 
![]() B (g) is2.5 × 10−2 . The rate constant of the forward reaction is 0.5 sec – Calculatethe rate constant of the reverse reaction
B (g) is2.5 × 10−2 . The rate constant of the forward reaction is 0.5 sec – Calculatethe rate constant of the reverse reaction![]() 2HI .The number of moles of H2 , I22
2HI .The number of moles of H2 , I22
FORM SIX CHEMISTRY EXAM SERIES 24
FORM SIX CHEMISTRY EXAM SERIES 24
Hub App
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256
 For Call,Sms&WhatsApp: 255769929722 / 255754805256
For Call,Sms&WhatsApp: 255769929722 / 255754805256